 BACTERIAL MENIGITIS
BACTERIAL MENIGITIS
Bacterial meningitis is the infection of the arachnoid membrane, subarachnoid space, and cerebrospinal fluid by bacteria
A total of c.3 million cases of meningitis worldwide
In Australia, menigitis incidence is about 9 cases per 100 000 person-years
Bacterial meningitis is diagnosed about half as much as viral
Incidence ranges from 65 cases/100,000 people (Africa) to 2/100000 (US/Canada)
Overall mean worldwide mortality is 16% but ranges from 2% – 33% depending on country
The subarachnoid space is bounded externally by the arachnoid membrane and internally by the pia, and dips into the brain along blood vessels in the perivascular (Virchow-Robin) spaces.
It extends from the optic chiasm to the cauda equina and surrounds the brain and spinal cord completely.
Head and neck infections (e.g. sinusitis, mastoiditis, otitis media)
Head and neck surgery or prostheses (e.g. cochlear implants, VP shunt, ICP monitor, EVD, craniectomy)
CSF leak (e.g. base of skull fracture)
Extremes of age (e.g. pneumococcus and listeria)
Comorbidities (e.g. liver and renal failure)
Immunosuppression (e.g. HIV, functional asplenia, splenectomy, hypogammaglobulinemia, complement deficiency, steroids, diabetes mellitus)
Malnutrition
Low socioeconomic status and overcrowding
Exposure to others with infective source (esp neisseria meningitidis)
STREP PNEUMONIAE
Strep Pneumoniae
Gram positive alpha or beta haemlolytic faculative anaerobe
Commonest cause of BM overall
Streptococcus pneumoniae, or pneumococcus
Gram positive cocci in pairs (diplococci) or chains. Alpha-haemolytic. Found in upper resp tract.
Treat with ceftriaxone (or cefotaxime) and vancomycin (some are resistant to cef) until sensitivities known.
NEISSERIA MENINGITIDIS
NEISSERIA MENINGITIDIS
Neisseria meningitidis or meningococcus
About 10% of adults are carriers of the bacteria in their nasopharynx.
As an exclusively human pathogen it is the main cause of bacterial meningitis in children and young adults, causing developmental impairment and death in about 10% of cases.
It causes the only form of bacterial meningitis known to occur epidemically, mainly in Africa and Asia: Historically a cause of large epidemics of meningitis due to N. meningitidis serogroup A in the “meningitis belt” of sub-Saharan Africa. Implementation of the conjugate vaccine has virtually eliminated these meningococcal epidemics. Trotter 2017
It occurs worldwide in both epidemic and endemic form.
N. meningitidis is spread through saliva and respiratory secretions during coughing, sneezing, kissing, chewing on toys and even through sharing a source of freshwater.
HAEMOPHILUS INFLUENZAE Type B
HAEMOPHILUS INFLUENZAE Type B
Now less common after HiB Vaccine
Small, pleomorphic gram negative cocco-bacilli. Can appear as filamentous rods in clinical specimens obtained from patients who have received beta-lactam antibiotics. Fastidious organism (has specific growth requirements) so ‘chocolate’ agar (brown agar with growth factors) required for growth in lab.
Once the most common cause of bacterial meningitis, widespread use of Hib conjugate vaccines in infancy has led to a dramatic decline in the incidence of invasive Hib disease in children.
Strains other than type b continue to cause occasional invasive infection (including meningitis) in children and adults.
Type B is the most virulent, accounting for vast majority of meningitis cases
ESCHERIA COLI
E.COLI
2nd commonest cause in neonates. From GU tract.
Gram negative rod (bacillus)
2nd commonest cause of BM in neonates; infection from mothers GU tract
Premature and low birth-weight babies are at much higher risk.
Also seen as nosocomial infection e.g. catheter associated ventriculitis
When E. coli infection occurs after six days of age, galactosemia (altered metabolism of galactose caused by a genetic enzyme deficiency) should be excluded.
GROUP B STREP
GROUP B STREP
Commonest cause BM in neonates. From GU tract.
Commonest cause of neonatal BM
AKA streptococcus agalactiae
Gram positive coccus but beta-haemolytic unlike pneumococcus which is alpha haemolytic
10%-30% of pregnant women carry the GBS; however the majority of neonates unaffected
Women with risk factors often given prophylactic ABx (penicillin or ampicillin) peripartum
Risk factors:
- Preterm labour <37+0 weeks (spontaneous or induced)
- Rupture of membranes (ROM) ≥18 hours prior to birth
- Maternal temperature ≥38 degrees intrapartum or within 24 hours of giving birth
- GBS colonisation in current pregnancy
- GBS bacteriuria in current pregnancy (any colony count)
- Previous baby with invasive GBS infection
LISTERIA MONOCYTOGENES
LISTERIA MONOCYTOGENES
Affects the old and neonates
Short gram-positive rod that occurs singly or in short chains. Challenging to identify on gram stain. May resemble gram positive diplococci (Strep pneumo), diphtheroids, or be gram variable and resemble haemophilus.
Causes meningoencephalitis
Rarer (<4%) cause of BM in neonates
Causes BM in immunosuppressed and elderly with high mortality rate
Most Listeria infections in adults are thought to result from oral ingestion of contaminated food, subsequent intestinal mucosal penetration and systemic infection
Resistant to cephalosporins so empirical ampicillin advised for meningitis in vulnerable populations (neonates, immunocompromised or age > 50)
 VIRAL CNS INFECTION
VIRAL CNS INFECTION
Meningitis is infection of the arachnoid membrane, subarachnoid space, and cerebrospinal fluid. Encephalitis is infection of the brain tissue. The presence or absence of normal brain function is the important distinguishing feature. The term ‘meningoencephalitis’ recognizes overlap between the two.
Most viruses are capable of causing either meningitis or encephalitis, but in general, a given virus will tend to cause one or the other (e.g HSV 1 – encephalitis, HSV 2 – meningitis)
Most common causes of aseptic meningitis include enteroviruses, HSV, HIV, West Nile virus, VZV, mumps, and lymphocytic choriomeningitis virus (LCMV).
Viral encephalitis can be either primary or postinfectious. HSV-1 is the most commonly identified cause of sporadic encephalitis in Australia. West Nile has emerged as the most common cause of viral encephalitis in the United States.
Most common causes are primary viral infection or immune mediated; this section just covers primary viral infection.
Viral meningitis is among the most common CNS infections, which accounts for the majority of cases of total viral CNS infections.
Encephalitis and myelitis, on the other hand, are much less common
The estimated annual incidence of all viral causes of encephalitis worldwide is
- 10 per 100,000 in children,
- 2 per 100,000 for adults and
- 6 per 100,000 for all ages
The mortality from viral encephalitis ranges from 3.8% to 7.4%
Causative virus only detected in minority of cases (40% in one study)
IMAGE MRI HSV:

HSV MRI Axial DWI
IMAGE CREDIT
Contrast-enhanced MRI is sensitive for early HSV encephalitis, showing edema in the orbitofrontal and temporal areas, which HSV typically infects.
MRI shows demyelination in progressive multifocal leukoencephalopathy and may show basal ganglia and thalamic abnormalities in West Nile and eastern equine encephalitis.
Children 5 times more likely to get than adults
Travel to area with high endemic rates
A detailed history is key, including:
> Sexual (e.g. HIV)
> Travel (e.g. West Nile, St Louis, Western and Eastern Equine, Japanese Encephalitis)
> Exposure history to insects (e.g. tick-borne, Nipah, Hendra, Zika)
> Exposure History to animals (e.g. rabies)
ENTEROVIRUS
ENTEROVIRUS
Commonest cause of viral CNS infections
Enterovirus CNS infections most commonly manifest as an aseptic meningitis.
Among infants, aseptic meningitis is most frequently due to group B coxsackie viruses and echoviruses.
Encephalitis occurs less frequently.
Most common cause of viral CNS infection – 1 billion people per year
Although infections are frequently asymptomatic, human enteroviruses can cause a variety of symptoms comprising fever, headache, respiratory illness, sore throat, and, occasionally, vomiting and diarrhea
HEV71 is a type of enterovirus that can cause large outbreaks of hand-foot-mouth disease (HFMD) and, in some children, meningitis, acute flaccid paralysis and a severe brainstem encephalitis with high mortality.
Coxsackieviruses and echoviruses are enteroviruses
HERPES SIMPLEX VIRUS
HERPES SIMPLEX VIRUS 1 & 2
PCR and MRI diagnostic
HSV1
> Mainly associated with infections of mouth (coldsores) and throat in early life but may also cause genital herpes infections.
> Frequently asymptomatic.
> Around 90% of adults become infected with HSV1 during their life
> The most common cause of sporadic viral encephalitis in adults.
> It is important to diagnose because it is treatable with acyclovir, and treatment success is related to early initiation of therapy.
HSV2
> Associated with genital herpes predominantly in adolescents and adults as it is transmitted through sexual activity.
> Tends to manifest as viral meningitis in immunocompetent adults, and usually has a good prognosis.
> The role of antiviral therapy for immunocompetent adults is unclear and may not be warranted.
> In neonates HSV-2 CNS infection is serious, manifesting as either skin, eye and mouth disease (45%), CNS disease (30%) or disseminated disease (25%) affecting multiple organs. Early treatment with acyclovir is indicated.
Herpes Encephalitis
> Virus is initially present initially in the limbic cortex.
> It may then spread to the adjacent frontal and temporal lobes of the brain.
> It is the destruction of tissue in these areas together with brain swelling from the inflammation, which causes many of the symptoms.
> Affects all ages, but is most common, and most severe, in children (33% <20y) and the elderly (50% >50y)
VARICELLA ZOSTER VIRUS
VARICELLA ZOSTER VIRUS
Chicken Pox and Shingles
VZV causes chicken pox (primary infection) and shingles (reactivation of latent VZV infection down a nerve root ganglia).
VZV can cause meningitis or encephalitis. Encephalitis more commonly complicates VZV infection in immunocompromised patients, but can more rarely be seen in previously healthy hosts.
Intravenous acyclovir should be administered to patients with neurologic complications of VZV where viral replication is thought to be playing a role.
VZV encephalitis causes a headache, fever, vomiting, and altered level of consciousness or even seizures.
Positive PCR testing in CSF confirms VZV
Risk factors:
- Age > 50
- Immunocompromise (reduced T cell-mediated immunity)
OTHER VIRUSES
OTHER VIRUSES
HIV, EBV, echovirus, Mumps, Rabies, arbovirus, CMV, poliovirus, Toscana virus
HIV: particular;y at time of seroconversion
EBV: More prevalent in some areas e.g. Middle East
Measles virus: acutely or subacute sclerosing panencephalitis can occur years after infection
JC virus: in immunosuppressed, esp HIV, causes progressive multifocal leukoencephalopathy
Mumps: Rare now in vaccinated populations
Rabies: in non-vaccinated, usually , arbovirus,
CMV: more common in immunosuppressed e.g haematology pts
Poliovirus: Only in countries where vaccination incomplete
Arboviruses e.g. Toscana virus in Mediterranean (more)
 NOSOCOMIAL CNS INFECTION
NOSOCOMIAL CNS INFECTION
Those of you who work in critical care areas with neurosurgical patients will probably see this type of CNS infection more than anything else.
No consensus on definition.
CDC definition:
EITHER
- organisms are cultured from the cerebrospinal fluid (CSF)
OR
- the patient has at least one sign or symptom of ventriculitis including:
- fever (> 38°C),
- headache
- stiff neck/meningeal signs
- cranial nerve signs
- irritability with no other recognized cause
AND at least ONE of the following:
(a) increased white cells + elevated protein + decreased glucose in CSF,
(b) organisms seen on Gram stain of CSF,
(c) organisms cultured from blood,
(d) positive laboratory test of CSF, blood, or urine,
(e) diagnostic single antibody titer (IgM) or four-fold increase in paired sera (IgG) for the pathogen,
and if the diagnosis is made antemortem, the physician institutes appropriate antimicrobial therapy
Common organisms:
- Staphylococcal aureus
- Coagulase negative staphylococci (CoNS)
- E.coli
- Pseudomonas
- Klebsiella
- Acinetobacter
Common organisms: Staphylococcal aureus and Coagulase negative staphylococci (CoNS)
Early infection with skin flora is the most common type of cerebrospinal fluid shunt infection; about half of all shunt infections are due to CoNS and about a third of cases are due to Staphylococcus aureus.
Diphtheroids (such as Cutibacterium [formerly Propionibacterium] acnes and Corynebacterium jeikeium) may also be pathogenic.
Later shunt infections, from distal end contamination (months after placement), are more likely to be caused by streptococci, gram-negative bacteria (including Pseudomonas aeruginosa), anaerobes, mycobacteria, and fungi.
“Acceptable” shunt infection rate is < 7% REF
Commonest Pathogens:
- Staph. epidermidis (coagulase-negative staph): 60–75% of infections (most common)
- Staph. aureus
- Gram-negative bacilli: 6–20% (may come from intestinal perforation)
- Candida species
Presentation:
- Non-specific syndrome: fever, nausea, headache, lethargy, anorexia, irritability.
- May also present as shunt malfunction
- Erythema and tenderness along shunt tubing may occur.
- Distal infection of ventriculoperitoneal shunts may mimic acute abdomen.
- S. epidermidis infections tend to be indolent (smoldering).
- GNB infections usually cause more severe illness.
- Abdominal findings are more common; main clinical manifestation is fever, usually intermittent and low grade.
CoNS
Coagulase Negative Staph Aureus
Commonest cause of nosocomial CNS infections
Previous known as Staph epidermidis
CoNS are commensals of human skin.
They have relatively low virulence but are increasingly recognized as significant pathogens when introduced into sterile sites but medical hardware.
Distinction between contamination and true infection is important for clinical management of CoNS. For this reason, cultures of the shunt or drain components are not recommended unless infection is suspected clinically.
Microbiologic factors favoring infection over contamination include growth in culture within 48 hours, and multiple cultures positive for the same organism with identical antibiograms and/or molecular evidence of genetically identical strains.
Resistance to methicillin and semisynthetic penicillins is observed in > 80 % of clinical CoNS isolates. CoNS are reliably sensitive to Vancomycin, so this is recommended for empiric therapy.
However, vancomycin has poor CSF penetration (1 percent in uninflamed meninges; 5 percent in inflamed meninges)
An “accidental” pathogen
Commonest cause of nosocomial infections
Colonises skin and mucous membranes
S. epidermidis belongs to the group of coagulase-negative staphylococci (CoNS), which is distinguished from coagulase-positive staphylococci such as S. aureus by lacking the enzyme coagulase
Treatment is complicated by specific antibiotic resistance genes and the formation of biofilms, multicellular agglomerations that have intrinsic resistance to antibiotics and mechanisms of host defense.
Even higher rates of methicillin resistance than staph aureus
Despite widespread resistance to methicillin and other antibiotics, most S. epidermidis-infected catheters may still be treated with antibiotics such as vancomycin.
Catheter removal is often required.
REFERENCE
STAPH AUREUS
STAPH AUREUS
Also very common cause of nosocomial infection
Gram positive coccus which colonized human skin and found in anterior nares of 10-40% of people.
Catalase positive (which differentiated it from streptococci and enterococci) and coagulase positive (which differentiates it from CoNS).
Major human pathogen
As a CNS infection, it is usually a hospital-acquired infection secondary to:
- Neurosurgical procedures
- Shunt devices
- Trauma
- Embolic spread from another ananatomical site
Treatment is flucloxacillin (or equivalent) for MSSA and vancomycin for MRSA. Oral rifampin is not routinely added to the above regimens but may augment treatment in the setting of refractory cases.
KLEBSIELLA PNEUMONIAE
KLEBSIELLA PNEUMONIAE
A rarer cause of nosocomial infections
Gram negative rods which are usually harmless colonisers of the human gut.
Organisms are capsular which may make bacilli look thicker than other gram-negative rods on gram stain, and give colonies a mucoid appearance on agar.
Rare cause of community acquired infection in immunocompetent hosts. Community acquired systemic infection with pyogenic liver abscesses and endophthalmitis more common in Taiwan and South Korea due to hypermucoid strains of the capsular K1 (or occasionally K2) serotype.
Common cause of nosocomial infection but rare cause of CNS infection. Generally only seen in context of late device related infections
Most Klebsiella inherently resistant to amoxicillin. Many now multi-drug resistant due to horizontal transmission of antibiotic resistance genes.
REF
 BRAIN ABSCESS
BRAIN ABSCESS
A good reason not to do an LP
Incidence 0.3 per 100 000
70% Male
Mean age 34
Cerebral abscesses result from pathogens growing within the brain parenchyma.
Initial parenchymal infection is known as cerebritis, which may progress into a cerebral abscess.
Historically direct extension from sinus or scalp infections was the most common source.
Now haematological spread is the most common source.
Direct introduction by trauma or surgery is rare.
Cerebral infection is commonly divided into four stages with distinct imaging and histopathologic features:
- Early cerebritis (Day 1-3: a focal infection without a capsule or pus formation,can resolve or develop into frank abscess)
- Late cerebritis (Day 4-9)
- Early abscess/encapsulation (Day 10-14)
- Late abscess/encapsulation (> Day 14: collagen capsule, necrotic centre, gliosis around capsule)
The direct spread of organisms from a contiguous site (e.g. from otitis media, mastoiditis) usually causes a single brain abscess.
Brain abscesses associated with bacteremia usually result in multiple abscesses that are most commonly located in the distribution of the middle cerebral artery.
Endocarditis and IV drug use
Pulmonary abscess
Pulmonary AVMs and AV fistulas
Congenital cyanotic heart disease
Immune compromise (especially HIV)
Chronic sinusitis/otitis
Dental procedures
Symptoms are similar to any other mass lesions but tend to progress rapidly:
- Headache
- Nausea / vomiting
- Lethargy
- Hemiparesis and seizures in 30-50% cases
Abscesses may rupture into the ventricular system, which results in a sudden and dramatic worsening of the clinical presentation and is a bad prognostic sign.
CT
Need pre- and post-contrast scans, unless going straight to MRI regardless of the CT findings. Typical appearances include:
- Outer hypodense and inner hyperdense rim (double rim sign) in most cases
- Ring of iso- or hyperdense tissue, typically of uniform thickness
- Central low attenuation (fluid/pus)
- Surrounding low density (vasogenic oedema)
- Ventriculitis may be present, seen as enhancement of the ependyma
- Obstructive hydrocephalus will commonly be seen when intraventricular spread has occurred
MRI
MRI is more sensitive than CT. Although peripherally-enhancing lesions may be non-specific by imaging, diffusion-weighted sequences (less commonly MR spectroscopy) showing central diffusion restriction are critical for suggesting the diagnosis of a cerebral abscess.
T1
- central low intensity (hyperintense to CSF)
- peripheral low intensity (vasogenic oedema)
- ring enhancement
- ventriculitis may be present, in which case hydrocephalus will commonly also be seen
T2/FLAIR
- central high intensity (hypointense to CSF, does not attenuate on FLAIR)
- peripheral high intensity (vasogenic oedema)
- the abscess capsule may be visible as an intermediate to slightly low signal thin rim
DWI/ADC
- high DWI signal is usually present centrally
- represents true restricted diffusion.
- peripheral or patchy restricted diffusion may also be seen; this finding is however not as constant as one may think, with up to half of the rim-enhancing lesions demonstrating some restriction not proving to be abscesses.
- in some immunocompromised states, central content may not diffusion restrict
SWI
- low-intensity rim
- complete in 75%
- smooth in 90%
- mostly overlaps with contrast-enhancing rim
- dual rim sign: a hyperintense line located inside the low-intensity rim
MR perfusion: rCBV is reduced in the surrounding oedema cf. to both normal white matter and tumour oedema seen in high-grade gliomas
MR spectroscopy: elevated peaks are seen corresponding to lipids/lactate, succinate, acetate, and amino acids (alanine, valine, leucine, and isoleucine)
Wide variety of organisms cause brain abscess.
The pathogens involved differ depending upon the site of the primary infection.
Sources associated with specific microorganisms:
> Paranasal sinuses – Streptococcus spp (especially S. milleri), Haemophilus spp, Bacteroides spp, Fusobacterium spp
> Odontogenic sources – Streptococcus spp, Bacteroides spp, Prevotella spp, Fusobacterium spp, Haemophilus spp
> Otogenic sources – Enterobacteriaceae, Streptococcus spp, Pseudomonas aeruginosa, Bacteroides spp
> Lungs – Streptococcus spp, Fusobacterium spp, Actinomyces spp
> Urinary tract – Pseudomonas aeruginosa, Enterobacter spp
> Penetrating head trauma – Staphylococcus aureus, Enterobacter spp, Clostridium spp
> Neurosurgical procedures – Staphylococcus spp, Streptococcus spp, Pseudomonas aeruginosa, Enterobacter spp
> Endocarditis – Viridans streptococci, S. aureus
> Congenital cardiac malformations (especially right-to-left shunts) – Streptococcus spp
> Immunocompetent hosts – usually bacterial species
> Immunocompromised – broad array including fungi
The immunocompromised patient is susceptible to a host of other organisms including:
- Toxoplasma gondii
- Listeria monocytogenes
- Nocardia asteroides
- Candida albicans
- Mycobacterium spp.
- Aspergillus fumigatus
- Fungi that cause mucormycosis
In terms of how common each organism is:
- Streptococcus sp: 35-50%, especially S. pneumoniae
- Sterile: 25%
- Mixed: variable, 10-90% of cases depending on source
- Staphylococcus aureus and epidermidis: following neurosurgery
- Gram-negative species more common in infants
- Listeria in pregnant women and older patients
- Group B Streptococcus (GBS) and E. coli in neonates
 SUBDURAL EMPYEMA
SUBDURAL EMPYEMA
Think of this if a patient has fever, headache, meningism and and lateralising signs like hemiparesis.
Again, avoid LP
A suppurative infection in the subdural space.
This has no anatomic barrier so can spread over the convexity and into the interhemispheric fissure.
Infection may spread to the subdural space from air sinuses or from the middle ear.
The subdural space is traversed by bridging arteries and veins but has no vascular network of its own.
Therefore, antibiotics have no access to this space.
The usual portal of entry is from the exterior.
If infection arises from ears or paranasal sinuses, likely to be streptococci and/or other anaerobes such as Cutibacterium (formerly Propionibacterium) and Peptostreptococcus species.
If infection follows neurosurgery, likely to be staphylococci, especially Staphylococcus aureus or gram-negative bacteria.
Infection can also spread inward from osteomyelitis of the skull or be introduced by fetal monitoring probes applied to the skull during birth.
Less common than cerebral abscess (ratio of abscess:empyema is ≈ 5:1).
Male:female ratio is 3:1.
Location: 70–80% are over the convexity, 10–20% are parafalcine.
Causes
Paranasal sinusitis (especially frontal): 70%
Otitis (usually chronic otitis media): 15%
Post surgical (neuro or ENT): 4%
Trauma: 3%
Meningitis (more common in peds): 2%
Others including unrelated infections: 6%
Suspect if meningismus and unilateral hemisphere dysfunction.
Marked tenderness to percussion or pressure over affected air sinuses is common
In order or frequency, symptoms are:
- Fever
- Headache
- Meningismus
- Hemiparesis
- Altered mental status
- Seizures
- Sinus tenderness, swelling or inflammation
- Nausea and/or vomiting
- Homonymous hemianopsia
- Speech difficulty
- Papilledema
CT with contrast
IV contrast is usually helpful.
Crescentic or lenticular extracerebral lesion with dense enhancement of medial membrane
As with any subdural collection, there is inward displacement of the gray-white interface, ventricular compression and possible effacement of basal cisterns
Unenhanced CT may miss small lesions. Lesion is hypodense (but denser than CSF)
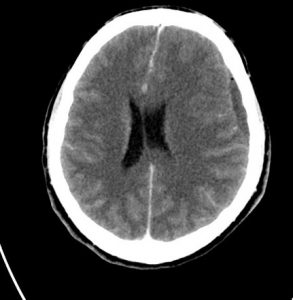
MRI
Low signal on T1WI, high signal on T2WI
Here is the (less common) parafalcine SDE:

T1 MRI SDE

T2 MRI SDE
If associated with sinusitis, often caused by aerobic and anaerobic streptococci
Following trauma or neurosurgical procedures, staphylococci and Gram-negative species predominate (Note: S. aureus rare)
Sterile cultures occur in up to 40%
Bugs in order of frequency:
- Aerobic streptococcus
- Staphylococci
- Microaerophilic and anaerobic strep
- Aerobic Gram-negative rods e.g. Escherichia coli, Klebsiella pneumoniae, Serratia marcescens.
- Other anaerobes
 EPIDURAL ABSCESS
EPIDURAL ABSCESS
Think of this if patients have back pain, fever, and spine tenderness
An epidural space filled with adipose tissue exists normally around the spinal cord.
A spinal epidural abscess arises when organisms from osteomyelitis or tuberculosis of the vertebral column spread to this space.
There is no epidural space normally in the cranium.
However, a cranial epidural abscess may develop when bacteria colonize a traumatic epidural hematoma, or when infection from air sinuses extends in the plane between the dura and bone.
Incidence: 0.2–1.2 per 10,000 hospital admissions annually
Average age: 58
Thoracic level is the most common site (≈ 50%), followed by lumbar (35%) then cervical (15%)
Often associated with vertebral osteomyelitis and intervertebral discitis.
Causes
No source can be identified in up to 50% of patients
Hematogenous spread is the most common source (26–50% of cases) either to the epidural space
or to the vertebra with extension to epidural space. Sources include:
- Skin infections (most common): furuncle may be found in 15% of cases
- Parenteral injections, especially with IV drug abuse
- Bacterial endocarditis
- UTI
- Respiratory infection (including otitis media, sinusitis, or pneumonia)
- Pharyngeal or dental abscess
Direct extension from:
- Decubitus ulcer
- Psoas abscess
- Penetrating trauma, including: abdominal wounds, neck wounds, GSW
- Pharyngeal infections
- Mediastinitis
- Pyelonephritis with perinephric abscess
- Dermal sinus
Following spinal procedures (e.g. epidurals or LP’s) or operations (lumbar discectomy)
After recent back trauma
All forms of immunosuppression, most commonly:
- Diabetes
- IV drug abuse
- Chronic renal failure
- Alcoholism
Usually presents with excruciating pain localized over spine with tenderness to percussion.
Radicular symptoms follow with subsequent distal cord findings, often beginning with bowel/bladder disturbance, abdominal distension, weakness progressing to para- and quadriplegia.
Average time is 3 days from back pain to root symptoms; 4.5 days from root pain to weakness; 24 hrs from weakness to paraplegia.
Fever, sweats or rigors are common, but are not always present
A furuncle (skin boil) somewhere on the body may be identified in 15%.
Patients may be encephalopathic
Meningismus with a positive Kernig sign may occur.
Patients with postoperative SEA may demonstrate surprisingly few signs or symptoms aside from local pain
MRI with Gadolinium is best
Also look for associated osteomyelitis and discitis
2 patterns:
- Phlegmonous stage of infection results in homogeneous enhancement of the abnormal area which correlate to granulomatous-thickened tissue with embedded micro-abscess without a significant pus collection
- Liquid abscess surrounded by inflammatory tissue which shows varying degree of peripheral enhancement with gadolinium

MRI T2

MRI T1 + C
Staph. aureus: the most common organism (cultured in > 50%)
Streptococcus: second most common
E.coli
Pseudomonas aeruginosa
Serratia marcescens
Enterobacter
Chronic infections:
- TB, usually associated with vertebral osteomyelitis,
- Brucellosis
- Fungal: cryptococcosis, aspergillosis
- Parasitic: Echinococcus
WHO GETS WHAT?
So just to recap, which patients are more likely to get which infections?
BACTERIAL MENINGITIS
Streptococcus Pneumoniae
Neisseria meningitidis
VIRAL MENINGITIS
Enterovirus
Herpes Simplex
Varicella Zoster
BACTERIAL MENINGITIS
Group B Streptococcus (streptococcus agalactiae)
E. Coli
Listeria Monocytogenes
VIRAL MENINGITIS
Enterovirus
Herpes Simplex
FUNGI
Aspergillus spp. – Brain Abscess
Candida spp. – Abscess
Cryptococcus neoformans – Meningoencephalitis
VIRUSES
Herpes simplex virus – meningitis, encephalitis
Varicella zoster virus – meningitis, encephalitis
Cytomegalovirus – meningitis, encephalitis
Epstein–Barr virus – meningitis, encephalitis
Human Herpesvirus – meningitis, encephalitis
JC virus – PML in HIV
West Nile Virus – encephalitis
MYCOBACTERIUM
Tuberculosis – meningitis, brain abscess, spinal epidural abscess
PARASITES
Toxoplasma gondii – brain abscess
BACTERIA
Any of the usual; higher risk of less common bacteria
Meningitis, cerebritis, brain abscesses, subdural empyema, spinal epidural abscess
Specifically think of:
Nocardia – Brain abscess
Listeria monocytogenes – meningitis, encephalitis
BACTERIAL MENINGITIS
Streptococcus Pneumoniae
Neisseria meningitidis
Listeria monocytogenes
Haemophilus influenzae
Group B Streptococcus
FUNGAL MENINGITIS
Cryptococcus neoformans
VIRAL MENINGITIS
Enterovirus
Herpes Simplex
Varicella Zoster
MYCOBACTERIUM
Mycobacterium Tuberculosis
EVD
Staphylococcal aureus
Coagulase Negative Staphylococcus aureus (CoNS)
E.coli
Pseudomonas
Klebsiella
Acinetobacter
VP SHUNT
CoNS
Staph. aureus
Gram-negative bacilli
Candida species
Variation exists around the world as to the most common CNS infections e.g.
Mediterranean
Toscana phlebovirus
SUB SAHARAN AFRICA
Neisseria meningitidis
Neurocysticercosis
Tuberculous spondylodiscitis
Tuberculous Meningitis/Osteomyelitis
Middle East
EBV encephalitis
Southeast Asia
Intracranial abscesses in general
TB prevalent Countries
Top 8: India, Indonesia, China, Philippines, Pakistan, Nigeria, Bangladesh, South Africa
TB meningitis, hard or soft granuloma, cerebritis, arachnoiditis, and spinal involvement all possible.
Taiwan
Klebsiella pneumoniae – bacterial meningitis
Streptococcus viridans – bacterial meningitis
Unvaccinated areas
Haemophilus influenzae
Rabies virus
Measles virus
Mumps virus
Polio virus
REFERENCE

 BACTERIAL MENIGITIS
BACTERIAL MENIGITIS



