Patterns and their Management
There are specific patterns of TBI which are relevant and they have specific management.
Of course more than one pattern can appear at once…
Extradural haematoma
 TAKE HOME POINTS
TAKE HOME POINTS
> EDH, also called EPIdural Haematoma
> Only 1% Head trauma admissions; Ratio of male:female = 4:1
> Lens shaped collection that doesn’t cross suture lines
> Not all require surgery (> 30 ml always do)
> Prognosis better if evacuated fast
![]()
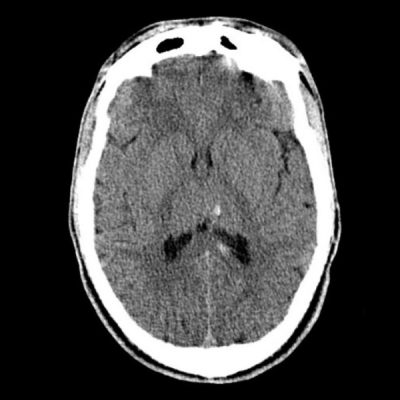
Lens shaped collection
Doesn’t cross suture lines
Mass effect
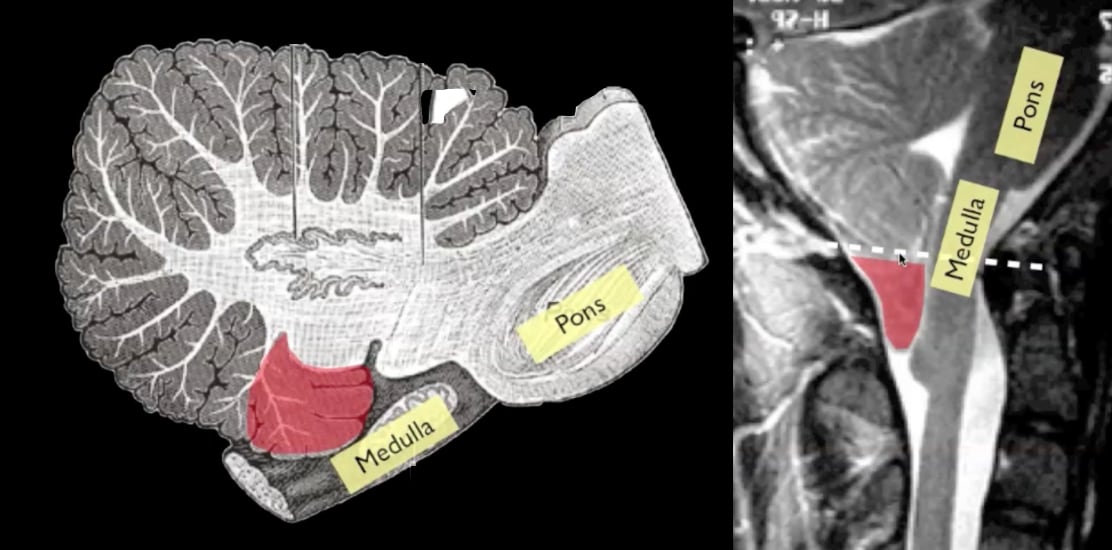
Temporal skull fracture
Classic location to damage middle meningeal artery
PATHOLOGY
> Classically, a temporoparietal skull fracture disrupts the middle meningeal artery as it exits its bony groove to enter the skull at the pterion, causing arterial bleeding (85%)
> Other bleeding bleeding sources are the middle meningeal vein or dural sinus
> 70% occur laterally over the hemispheres with their epicenter at the pterion, the rest occur in the frontal, occipital, and posterior fossa.
CLASSIC HISTORY
> Clear history of head trauma (unlike SDH’s).
> Often young patients involved in a head strike who may or may not lose consciousness transiently.
> Following the injury, they have a “lucid interval” where they regain a normal level of consciousness but have ongoing headache.
> Over the next few hours, they gradually lose consciousness, develop contralateral hemiparesis and ipsilateral pupillary dilatation.
IMAGING
Usually don’t cross suture lines
> EDH is a subperiosteal haematoma located on the inside of the skull, between the inner table of the skull and parietal layer of the dura mater (which is the periosteum).
> Classically lens shaped (convex), or “Idli” shaped.
> As a result, EDHs are usually limited in their extent by the cranial sutures, as the periosteum crosses through the suture continuous with the outer periosteal layer.
> This helps distinguish from SDH which are not limited by sutures.
MANAGEMENT
Indications for craniotomy and drainage:
EDH volume* > 30 cm3 should be evacuated regardless of GCS.
EDH with ALL of the following may be managed non-surgically with serial CT scans & close neurological observation in a neurosurgical centre:
- volume* < 30 cm3
- and thickness < 15 mm
- and midline shift (MLS) < 5 mm
- and GCS > 8
- and no focal neurologic deficit
*Volume is measured with the ABC/2 method
Outcomes
> Overall: 20–55% (higher rates in older series).
> Optimal diagnosis and treatment within a few hours results in 5–12% mortality.
> Mortality in patients without lucid interval is double that for patients with a lucid interval.
> Bilateral Babinski’s or decerebration before surgery carries worse prognosis.
> Death is often from respiratory arrest (uncal herniation causing injury to the midbrain)
> 20% of patients with EDH on CT also have ASDH at autopsy or operation.
> Mortality with both lesions concurrently is higher.
Subdural haematoma
 TAKE HOME POINTS
TAKE HOME POINTS
> SDH, also called Subdural Haemorrhage
> A collection of blood accumulating in the subdural space, the potential space between the dura and arachnoid mater of the meninges
> Two types – high force TBI with damaged underlying cortex and elderly pts with delicate bridging veins
> Twice as common as EDH
> Overall prognosis worse than EDH
![]()
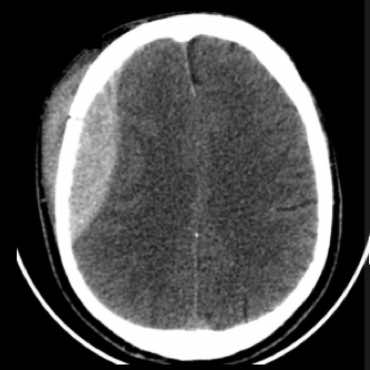
Acute traumatic SDH
Significant underlying brain injury
Underlying haemorrhagic contusion
3 mm midline shift
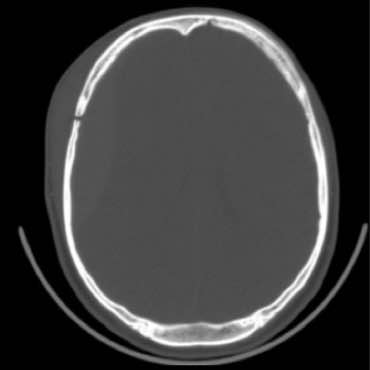
Acute on chronic bilateral SDH
Right bigger than left
No underlying brain injury evident
No midline shift
Hyperdense older blood above hypodense newer blood
PATHOLOGY
2 main types:
-
Accumulation around parenchymal laceration (usually frontal or temporal lobe). There is usually severe underlying primary brain injury.
-
Surface or bridging vessel torn from cerebral acceleration-deceleration during fast head motion. Often elderly patients. Anticoagulants contribute.
CLASSIC HISTORY
> For younger patients, usual context is high-force trauma. The SDH overlies a parenchymal injury and is a severe brain injury. There is no “lucid interval”.
> For elderly patients, there is often no good history trauma and relatively minor force can tear veins in the SD space. Anticoagulants are often contributory. There can be a lucid interval before decrease in consciousness and neurological deficits. There is less underlying brain injury in this context.
IMAGING
A crescent (concave) shape that is not limited by skull sutures.
Usually unilateral in adults
As haematoma ages, it darkens:
- Days 1-3 blood on CT is hyperdense (white)
- Days 4-14 roughly isodense (similar to brain)
- Days > 14 hypodense (darker than brain)
Layering of different aged blood may be visible (older dark blood often rises to top) REF
Management
Monitor ICP in all patients with ASDH and GCS < 9
Indications for surgery:
ASDH with thickness > 10 mm or midline shift (MLS) > 5mm (on CT) should be evacuated regardless of GCS
ASDH with thickness < 10 mm and MLS < 5 mm should undergo surgical evacuation if:
- GCS drops by ≥ 2 points from injury to admission
- and/or the pupils are asymmetric or fixed and dilated
- and/or ICP is > 20 mm Hg
Timing of surgery
ASDH meeting surgical criteria should be evacuated ASAP REF
Outcomes
Overal mortality 50-90%
Risk factors for worse outcome:
> Anticoagulated
> High force injury
> Age
> GCS on admission
Chronic SDH
Really a different condition to acute TBI
Usually caused by minor trauma in elderly pts as described above, although this is only identified 50% of the time
Bilateral in c.25%
Classically contain dark “motor oil” that does not clot (blood mixed with CSF)
If the subdural fluid becomes clear (CSF), it is called a subdural hygroma
Only treated if symptomatic (usually > 1cm maximal thickness) e.g. focal deficit, mental status changes, seizures
OR progressive increase in size on serial imaging (CT or MRI scans)
Often wait c. 2 weeks before draining if neurology / SDH size stable
Treatment either:
> Surgical evacuation of the haematoma
> Endovascular embolisation of the middle meningeal artery
Traumatic IntraCerebral haematoma
 TAKE HOME POINTS
TAKE HOME POINTS
> AKA haemorrhagic contusion AKA intraparenchymal haematoma
> Common in the setting of severe TBI
> Often associated with haemorrhage in other compartments
> Often considered as high density areas on CT
> TICH usually produce much less mass effect than their apparent size.
> Most commonly occur in areas where sudden deceleration of the head causes the brain to impact on bony prominences (e.g. temporal, frontal, and occipital poles) in coup or contrecoup pattern.
> TICH often enlarge (“blossom” and/or coalesce with time as seen on serial CTs.
> They also may appear in a delayed fashion
> Surrounding low density may represent associated cerebral oedema.
> CT scans months later often show surprisingly minimal or no encephalomalacia REF
![]()
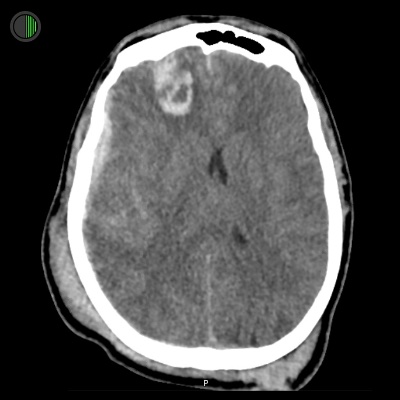
51M Fall from 4m
Right frontal TICH 25 x 25 x 35 mm
Right convexity hyperdense SDH measuring up to 5 mm
4.5 mm of midline shift to the left
The right lateral ventricle is almost completely effaced
Subarachnoid haemorrhage overlying the frontal lobes
Large scalp haematoma
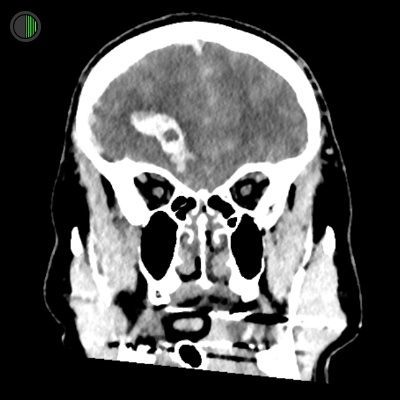
Same 51M: Fall from 4m
Can see height of right frontal TICH and the inferior position
Associated haemorrhage in other compartments
Right convexity hyperdense SDH visible here too
4.5 mm of midline shift to the left
Subarachnoid haemorrhage also visible here
PATHOLOGY
Most commonly occur in areas where sudden deceleration of the head causes the brain to impact on bony prominences (e.g. temporal, frontal, and occipital poles) in coup or contrecoup fashion.
CLASSIC HISTORY
Occur from high-force trauma
Typical causes include motor vehicle accidents or situations in which the head strikes the ground.
IMAGING
Can be anywhere but predictable from direction of skull strike and intrinsic shape of the skull cavity.
- Anterior cranial fossa floor
- Temporal pole
- Coup and contrecoup pattern
Often grow and merge (blossom) in the first 48 hours.
Mature over some weeks, initially appearing as haemorrhagic foci, followed by the development of surrounding oedema, before gradually fading away leaving behind more or less distinct areas of gliosis.
Surrounding low density may represent associated cerebral edema.
CT scans months later often show surprisingly minimal or no encephalomalacia REF
Management
Indications for surgical evacuation for TICH:
> Progressive neurological deterioration referable to the TICH, medically refractory IC-HTN or signs of mass effect on CT
> TICH volume > 50cm3 cc or ml
> GCS = 6–8 with frontal or temporal TICH volume > 20 ml with midline shift (MLS) ≥ 5 mm and/or compressed basal cisterns on CT
Non-operative management with intensive monitoring and serial imaging may be used for TICH without neurologic compromise and no significant mass effect on CT and controlled ICP
Traumatic SubArachnoid Haemorrhage
 TAKE HOME POINTS
TAKE HOME POINTS
> Common in TBI (c.35%)
> Commonest cause of SAH (more than aneurysmal)
> Better prognosis than aSAH
> Sometimes need to exclude aSAH as a cause of the trauma
> Presence is part of the Rotterdam Score implying worse prognosis than no tSAH
> There is no indication for nimodipine following tSAH
> There is no specific management for tSAH
![]()
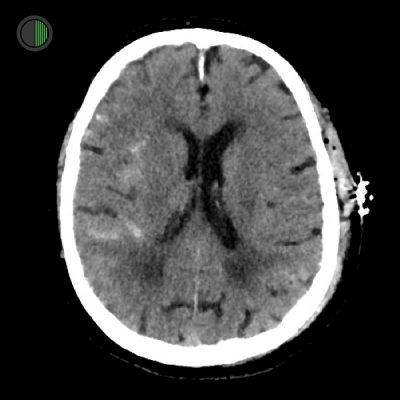
Traumatic SAH
Right Sylvian fissure subarachnoid haemorrhage
Scalp haematoma on contralateral side
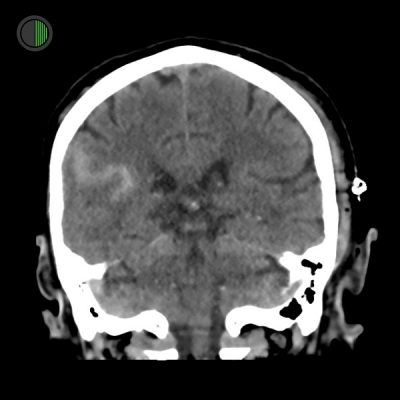
Traumatic SAH also visible in coronal
Right Sylvian fissure subarachnoid haemorrhage in “S” shape
Scalp haematoma on left
PATHOLOGY
More commonly seen in the cerebral sulci than in the Sylvian fissure and basal CSF cisterns
When in the basal cisterns, it has an affinity for the quadrigeminal cistern and ambient cistern
Also commonly seen adjacent to skull fractures and cerebral contusions.
Causes:
- Direct extravasation of blood from an adjacent cerebral contusion
- Arterial dissection
- Direct damage to small veins or arteries
- Sudden increase in intravascular pressures leading to rupture REF
CLASSIC HISTORY
Occur from high-force trauma
Typical causes include motor vehicle accidents or situations in which the head strikes the ground.
IMAGING
The distribution and amount of blood varies depending on underlying mechanism between patients
Often a small amount of blood is seen filling a few sulci, sometimes with an adjacent cerebral contusion.
Small amounts of blood may be seen in the interpeduncular fossa, appearing as a small hyperdense triangle, or within the occipital horns of the lateral ventricles.
Think about possibility of a ruptured aneurysm causing the trauma and perform angiography if appropriate REF
Traumatic IntraVentricular Haemorrhage
 TAKE HOME POINTS
TAKE HOME POINTS
> May cause obstructive hydrocephalus and require CSF drainage (EVD, potentially a shunt later)
> A Marker of severity in TBI in Rotterdam score
![]()
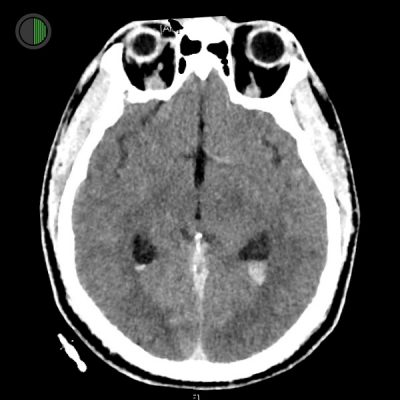
36M high speed MVA
Traumatic IVH
Layering of acute blood in posterior horns of lateral ventricles
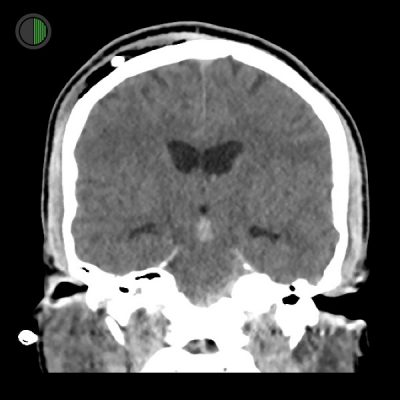
36M High speed MVA
Traumatic IVH
Blood visible in 3rd ventricle
Dilated ventricles consistent with hydrocephalus
Part of EVD visible on right
Scalp haematoma and air on right, around EVD
PATHOLOGY
Blood in ventricles
CLASSIC HISTORY
Severe TBI
IMAGING
Blood in the ventricles is hyperdense, heavier than CSF and so pools dependently, best seen in the occipital horns.
Acutely, if the volume is significant blood can fill the ventricle, and clot forming a ‘cast’.
There is often obstructive hydrocephalus; look for this specifically:
> In the lateral ventricles with enlargement of the temporal horns (best indicator)
> In the third ventricle with outward bowing of the lateral walls and inferior bowing of the floor
> Look for transependymal oedema visible as low-density change on CT around the margins of the ventricles (or high T2 signal on MRI)
In elderly patients or those with previous brain injury, don’t be tricked by chronically large ventricles looking like HCP.
This is called “hydrocephalus ex vacuo”
Management
CSF diversion if significant obstructive hydrocephalus
> Initially and EVD
> If persistent, more permanent CSF diversion is sometimes needed e.g. a ventriculoperitoneal shunt
Diffuse Axonal Injury
 TAKE HOME POINTS
TAKE HOME POINTS
> Seen after very severe TBI
> CT notoriously insensitive at detecting
> MRI much more sensitive
> Treatment is supportive
> Diagnosis important prognostically
![]()
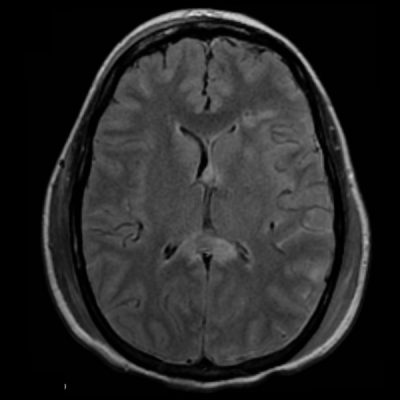
28M High speed MVA
Oedema and haemorrhage in corpus callosum REF
PATHOLOGY
Diffuse axonal injury is the result of shearing forces, typically from rotational acceleration (most often a deceleration).
Due to the slightly different specific gravities (relative mass per unit volume) of white and grey matter, shearing due to change in velocity occurs in axons at the grey-white matter junction.
These forces result in damage to the cells and result in oedema.
Actual complete tearing of the axons is only seen in severe cases.
CLASSIC HISTORY
High force TBI typically with rotational acceleration-deceleration
IMAGING
CT has low sensitivity at detecting DAI only detecting 19% of non-haemorrhagic DAI lesions (MRI 92%) REF
Small haemorrhagic lesions on CT may represent significant damage, with disproportionate oedema and clinical consequences.
Can have DAI with essentially normal looking CTB
Can grade DAI
> Grade 1 – Lobar: usually parasagittal regions of frontal lobes, periventricular temporal lobes
> Grade 2 – Callosal: usually posterior body and splenium of corpus callosum
> Grade 3 – Brainstem: dorsolateral midbrain, upper pons, and superior cerebellar peduncles REF
Management
> Supportive
> Diagnosis helpful for prognostication
> Initially and EVD
> If persistent, more permanent CSF diversion is sometimes needed e.g. a ventriculoperitoneal shunt
Herniation Syndromes
TBI can produce some herniation syndromes which present in different ways and may require different management.
With permission from Andy Neill
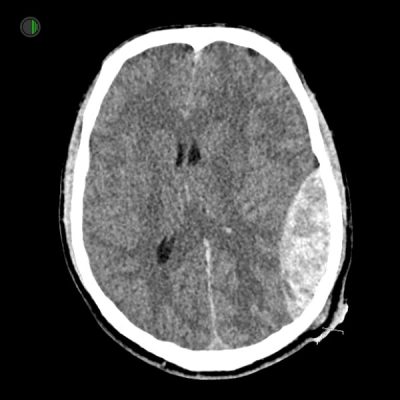
Traumatic EDH
Subfalcine herniation
12 mm midline shift
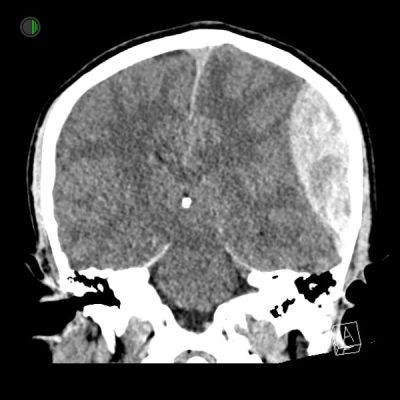
Traumatic EDH
Subfalcine herniation
12 mm midline shift
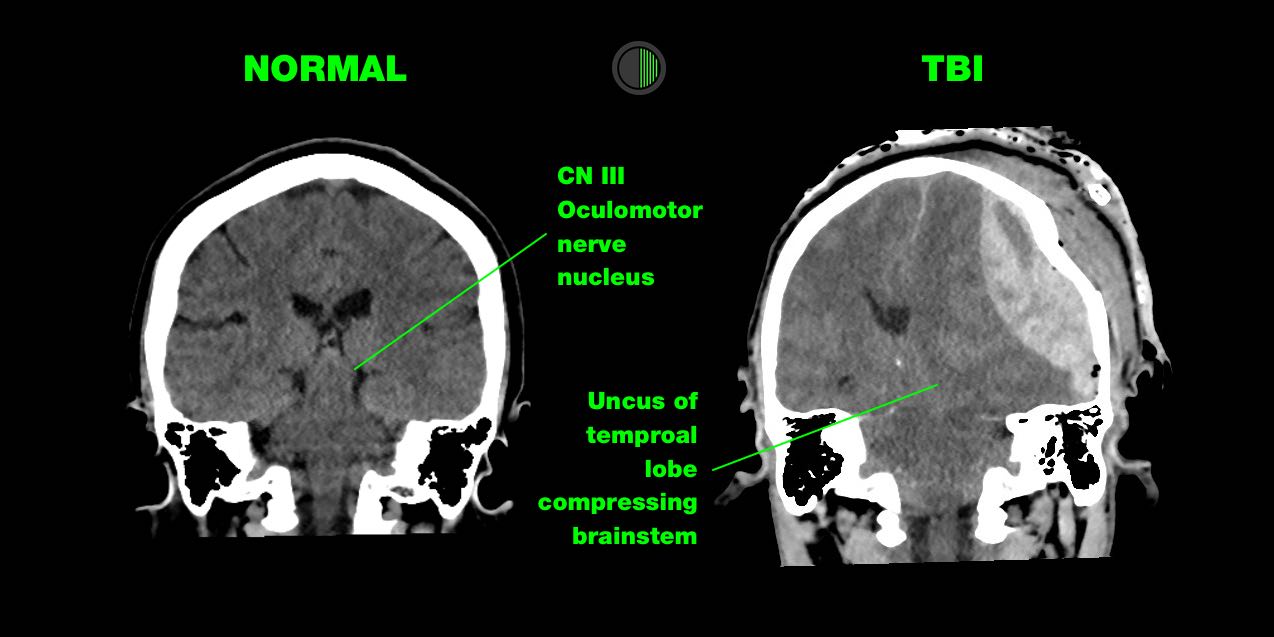
Sign of a neurosurgical emergency that requires immediate treatment